Neuromuscular Disease Therapeutics Market | Gene Therapy & Biologics Driving Market Expansion | DataM Intelligence
Explore trends and opportunities in the Neuromuscular Disease Therapeutics Market, including gene therapies and global regional analysis.
From Spinraza to Zolgensma, the Neuromuscular Disease Therapeutics Market is expanding rapidly, driven by innovative therapies, early diagnosis, and emerging market opportunities.”
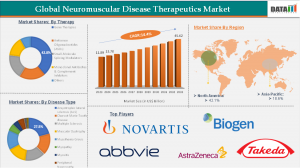
AUSTIN, TX, UNITED STATES, October 7, 2025 /EINPresswire.com/ -- According to DataM Intelligence, the neuromuscular disease therapeutics market was valued at US$ 11.89 billion in 2023, increased to US$ 13.70 billion in 2024, and is projected to reach US$ 45.62 billion by 2033, growing at a CAGR of 14.4% during the forecast period (2025–2033). The rising prevalence of neuromuscular diseases such as Spinal Muscular Atrophy (SMA), Duchenne Muscular Dystrophy (DMD), and Amyotrophic Lateral Sclerosis (ALS), coupled with increased healthcare spending and regulatory support for orphan drugs, has accelerated market expansion. Growing investments in genetic medicine and the commercialization of high-impact treatments such as Spinraza, Zolgensma, Evrysdi, and Elevidys are reshaping the therapeutic landscape. Furthermore, advancements in diagnostics and newborn screening programs have significantly improved early detection, allowing for timely intervention and better patient outcomes.— DataM Intelligence
Among various product categories, gene therapies lead the market with approximately 42.8% market share, owing to their ability to address the root genetic causes of disease. Regionally, North America dominates with about 42.1% share in 2024, attributed to a strong R&D base, advanced healthcare infrastructure, favorable regulatory policies, and early adoption of innovative treatments.
𝗚𝗲𝘁 𝗮 𝗦𝗮𝗺𝗽𝗹𝗲 𝗣𝗗𝗙 𝗕𝗿𝗼𝗰𝗵𝘂𝗿𝗲 𝗼𝗳 𝘁𝗵𝗲 𝗥𝗲𝗽𝗼𝗿𝘁 (𝗨𝘀𝗲 𝗖𝗼𝗿𝗽𝗼𝗿𝗮𝘁𝗲 𝗘𝗺𝗮𝗶𝗹 𝗜𝗗 𝗳𝗼𝗿 𝗮 𝗤𝘂𝗶𝗰𝗸 𝗥𝗲𝘀𝗽𝗼𝗻𝘀𝗲): https://www.datamintelligence.com/download-sample/neuromuscular-disease-therapeutics-market
Key Highlights from the Report:
➤ The global neuromuscular disease therapeutics market reached US$ 11.89 billion in 2023 and is expected to hit US$ 45.62 billion by 2033, growing at a CAGR of 14.4%.
➤ Gene therapies account for the largest share of approximately 42.8%, driven by strong efficacy and long-term benefits.
➤ North America leads the global market with a 42.1% share in 2024, supported by robust healthcare systems and early adoption of new therapies.
➤ SMA and DMD are the most prominent disease segments due to high diagnosis rates and successful commercial launches.
➤ Leading companies include Novartis, Biogen, AbbVie, Sarepta, AstraZeneca, and Argenx, reflecting intense competition and innovation.
➤ Key challenges include therapy affordability, manufacturing complexities, and limited access in emerging economies.
Recent Developments:
United States:
1. In July 2025, Biogen announced the FDA approval of a novel antisense therapy for Duchenne Muscular Dystrophy, improving patient mobility.
2. In June 2025, Sarepta Therapeutics expanded clinical trials for its gene therapy targeting rare neuromuscular disorders, including spinal muscular atrophy.
Japan:
1. In August 2025, Takeda Pharmaceutical initiated early-stage trials of enzyme replacement therapy for hereditary neuromuscular diseases.
2. In May 2025, Chugai Pharmaceutical partnered with academic hospitals to develop innovative biologics for myasthenia gravis and related disorders.
Company Insights
• Novartis Pharmaceuticals Corporation
• Biogen Inc.
• AbbVie Inc.
• AstraZeneca Plc
• Argenx SE
• Sarepta Therapeutics, Inc.
• Takeda Pharmaceutical Company Limited
• Nippon Shinyaku Co., Ltd.
• Corium, LLC
Market Segmentation:
The neuromuscular disease therapeutics market is segmented by product type, indication, route of administration, end-user, and region, offering a comprehensive view of the industry’s growth potential.
➤ By Product Type
Gene Therapies dominate with over 42% market share, revolutionizing treatment by targeting the underlying genetic mutations instead of merely alleviating symptoms. Treatments like Zolgensma and Elevidys exemplify how gene transfer can restore or replace defective genes, offering durable results with fewer doses.
Monoclonal antibodies, antisense oligonucleotides (ASOs), and RNA-based therapeutics are also expanding rapidly, addressing diseases previously untreatable through conventional drugs. Meanwhile, small molecules continue to serve as vital symptomatic treatments, particularly in managing disease progression and pain.
➤ By Indication
Spinal Muscular Atrophy (SMA) and Duchenne Muscular Dystrophy (DMD) are leading disease categories, accounting for the majority of market revenue. Both have seen multiple therapy approvals and increased patient awareness.
Other disorders such as Myasthenia Gravis, Amyotrophic Lateral Sclerosis (ALS), and Neuromyelitis Optica Spectrum Disorder (NMOSD) are gaining attention as drug pipelines expand and research funding grows.
➤ By Route of Administration
Therapies are delivered through intravenous, intrathecal, subcutaneous, and oral routes. Gene therapies often rely on intrathecal or intravenous delivery due to vector-based administration needs, whereas small molecule and antibody-based therapies commonly use oral or subcutaneous routes for improved patient compliance.
➤ By End-User
End-users include hospitals, neurology clinics, and specialty centers that manage rare diseases. The hospital segment holds a major share due to its infrastructure for advanced gene therapy administration and patient monitoring. The rise of home-based care and telemedicine is also improving treatment accessibility and adherence.
Looking For A Detailed Full Report? Get it here: https://www.datamintelligence.com/buy-now-page?report=neuromuscular-disease-therapeutics-market
Regional Insights:
North America
North America holds the largest market share, driven by strong investments in biotechnology, favorable reimbursement frameworks, and the presence of leading pharmaceutical innovators. The United States accounts for the majority of global sales due to early approvals, high patient awareness, and government-supported newborn screening programs. Canada is also experiencing growth with increasing access to orphan drugs and expanded healthcare coverage.
Europe
Europe is the second-largest region in this market, propelled by regulatory initiatives such as the European Medicines Agency’s (EMA) orphan drug incentives. Countries like Germany, France, and the UK have adopted gene therapy protocols and improved access pathways. However, disparities in reimbursement policies across European nations continue to affect adoption rates.
Asia-Pacific
Asia-Pacific represents the fastest-growing regional market. Rising healthcare investments in countries like Japan, China, and India, coupled with growing genetic testing awareness, are fueling rapid expansion. Japan leads in early adoption of advanced therapeutics, while China is investing heavily in domestic biopharmaceutical R&D. The region’s large population base and growing diagnostic infrastructure make it a future hotspot for market growth.
Latin America, Middle East & Africa
These regions exhibit gradual but steady growth. Government initiatives promoting rare disease treatment, alongside partnerships with global pharmaceutical firms, are improving access. However, high treatment costs and limited infrastructure remain key barriers to widespread adoption.
Market Dynamics
◆ Market Drivers
The neuromuscular disease therapeutics market is being propelled by several factors:
o Breakthrough Approvals: Therapies like Zolgensma, Evrysdi, and Spinraza have demonstrated unprecedented efficacy, leading to widespread clinical and commercial success.
o Advancements in Genetic Diagnostics: Early identification through newborn screening and genetic sequencing enables timely treatment and improved outcomes.
o Regulatory Incentives: Orphan drug designations, fast-track approvals, and priority review processes are encouraging rapid innovation.
o Rising Awareness and Advocacy: Global patient organizations are driving awareness, fundraising, and policy reforms to improve access to advanced therapies.
o Technological Innovation: The development of viral vectors, improved RNA editing technologies, and next-generation delivery systems are enhancing treatment precision.
◆ Market Restraints
Despite rapid progress, the market faces notable challenges:
o High Treatment Costs: Gene therapies can exceed several million dollars per patient, limiting affordability and reimbursement acceptance.
o Manufacturing Challenges: Scaling viral-vector production remains complex, with significant quality control and safety considerations.
o Limited Long-term Data: While short-term results are promising, long-term durability and safety of new therapies require continued monitoring.
o Regulatory and Ethical Issues: Differing global regulatory standards and ethical considerations around gene modification impact approval timelines.
o Access Disparities: Developing economies face significant gaps in diagnostic infrastructure and access to high-cost therapeutics.
◆ Market Opportunities
The market presents multiple high-potential opportunities:
o Emerging Markets Expansion: Improved healthcare spending and diagnostic capacity in Asia-Pacific, Latin America, and the Middle East offer new growth avenues.
o Next-generation Therapies: Innovations such as RNA editing, non-viral gene delivery, and exon-skipping techniques promise safer and more affordable solutions.
o Personalized Medicine: Tailoring therapies to patient-specific mutations enhances efficacy and reduces side effects.
o Collaborative Research: Partnerships between biotech firms and academic research centers are accelerating innovation and product approvals.
o Value-based Reimbursement Models: Novel payment systems such as outcome-based contracts are helping mitigate cost barriers.
Get Customization in the report as per your requirements: https://www.datamintelligence.com/customize/neuromuscular-disease-therapeutics-market
Reasons to Buy the Report:
✔ Comprehensive coverage of the global market size, growth projections, and segment-level insights through 2033.
✔ Strategic analysis of therapeutic modalities including gene therapy, biologics, and small molecules.
✔ Detailed regional breakdowns highlighting mature and emerging markets for investment planning.
✔ Competitive profiling of major companies and recent product approvals.
✔ Insights into regulatory frameworks, challenges, and growth opportunities for strategic decision-making.
Frequently Asked Questions (FAQs):
◆ How Big is the Neuromuscular Disease Therapeutics Market?
The market was valued at US$ 11.89 billion in 2023 and is projected to reach US$ 45.62 billion by 2033, growing at a CAGR of 14.4%.
◆ Which Product Segment Leads the Market?
Gene therapies dominate the market with over 42% share due to their ability to correct underlying genetic defects.
◆ What is the Forecasted Growth Rate for the Market?
The market is expected to grow at a CAGR of approximately 14.4% from 2025 to 2033.
◆ Which Region is Expected to Dominate During the Forecast Period?
North America is expected to maintain dominance owing to strong research infrastructure, early approvals, and favorable reimbursement policies.
◆ Who are the Key Players in the Neuromuscular Disease Therapeutics Market?
Key companies include Novartis, Biogen, AbbVie, AstraZeneca, Sarepta, Argenx, Takeda, and Nippon Shinyaku.
Conclusion:
The neuromuscular disease therapeutics market is undergoing a paradigm shift, fueled by gene-based innovations, improved diagnostic tools, and government support for rare disease treatments. North America continues to lead, while Asia-Pacific is emerging as the next growth powerhouse. Despite high costs and complex manufacturing processes, technological progress and evolving reimbursement models promise to make life-changing treatments more accessible. The coming decade will likely redefine patient care in neuromuscular disorders, with global collaborations and personalized medicine paving the way toward a more inclusive and effective therapeutic future.
Request for 2 Days FREE Trial Access: https://www.datamintelligence.com/reports-subscription
Power your decisions with real-time competitor tracking, strategic forecasts, and global investment insights all in one place.
Competitive Landscape
Sustainability Impact Analysis
KOL / Stakeholder Insights
Unmet Needs & Positioning, Pricing & Market Access Snapshots
Market Volatility & Emerging Risks Analysis
Quarterly Industry Report Updated
Live Market & Pricing Trends
Import-Export Data Monitoring
Have a look at our Subscription Dashboard: https://www.youtube.com/watch?v=x5oEiqEqTWg
Related Reports:
Adalimumab Market
Celiac Disease Drugs Market
Sai Kiran
DataM Intelligence 4market Research LLP
877-441-4866
email us here
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.